Home / Elixir R&D
Over 1000+ Users and 100+ Brands Trust GreyB































What's Elixir is offering?

Critical updates
Efficient Tracking and Analysis
Collaboration Opportunities
Centralized Platform

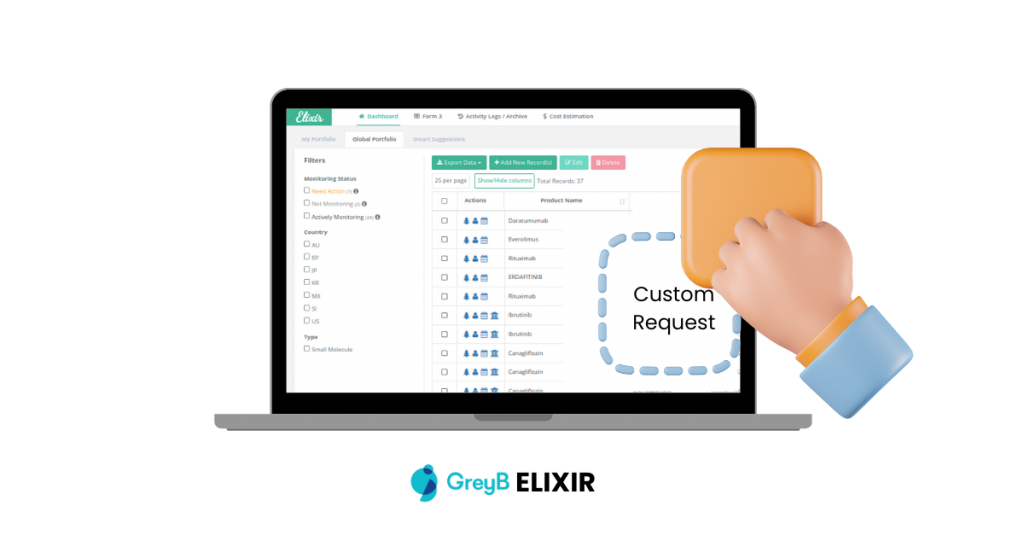
Customizable
What does Elixir Monitor?
Patents and their Divisionals/Continuations/Continuations-In- Parts protecting the Target drug
Patent Term Extension/SPCs/Third Party Opposition (TPO)
Monitors competitors through citations
Paragraph IV Litigation
Identifies 180 days of market exclusivity opportunities
Proceedings before the Unified Patent Court (UPC)
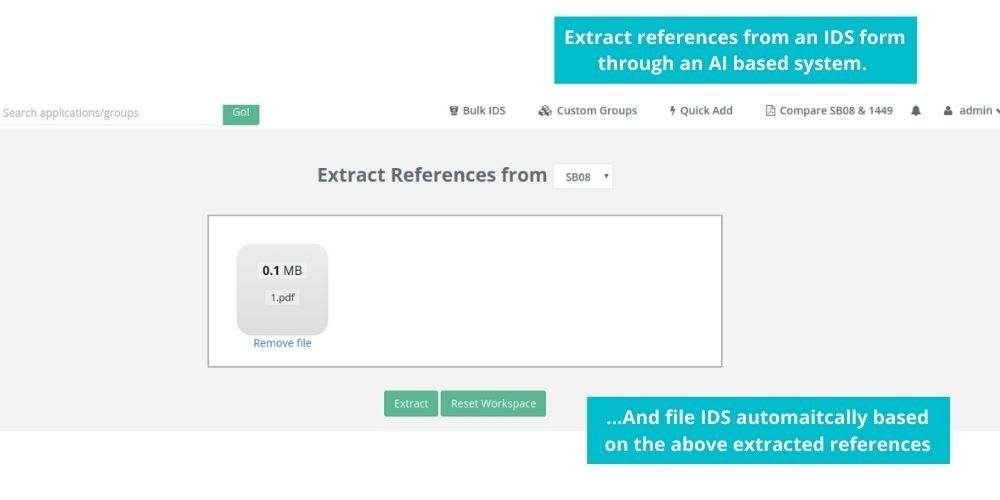
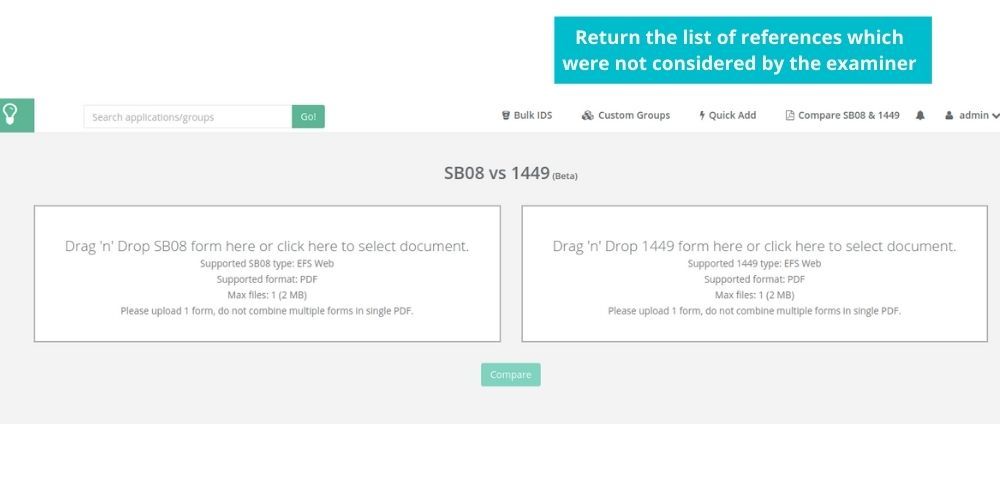
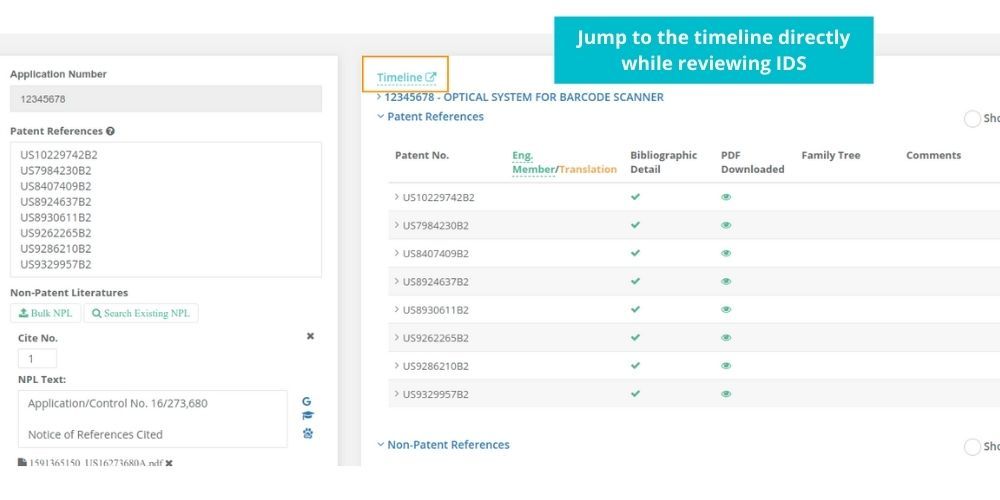
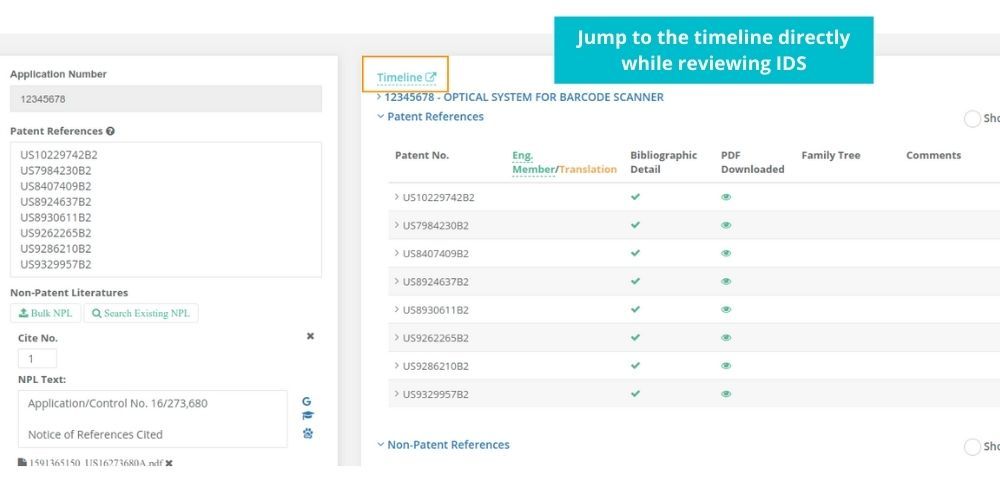
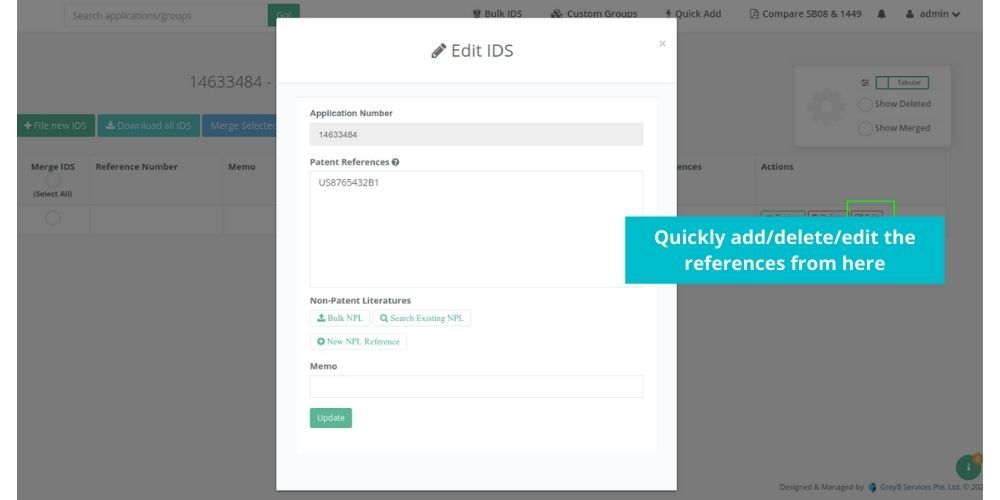
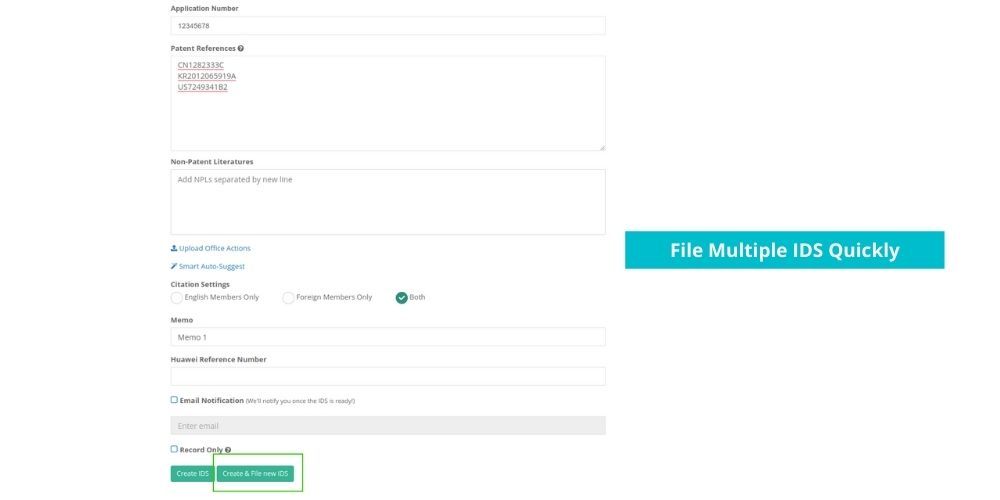
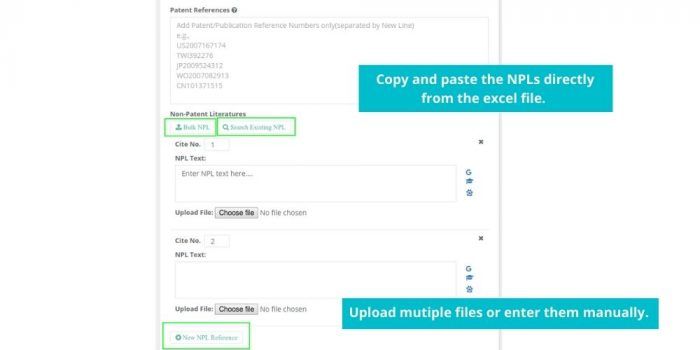
See how Elixir can help you
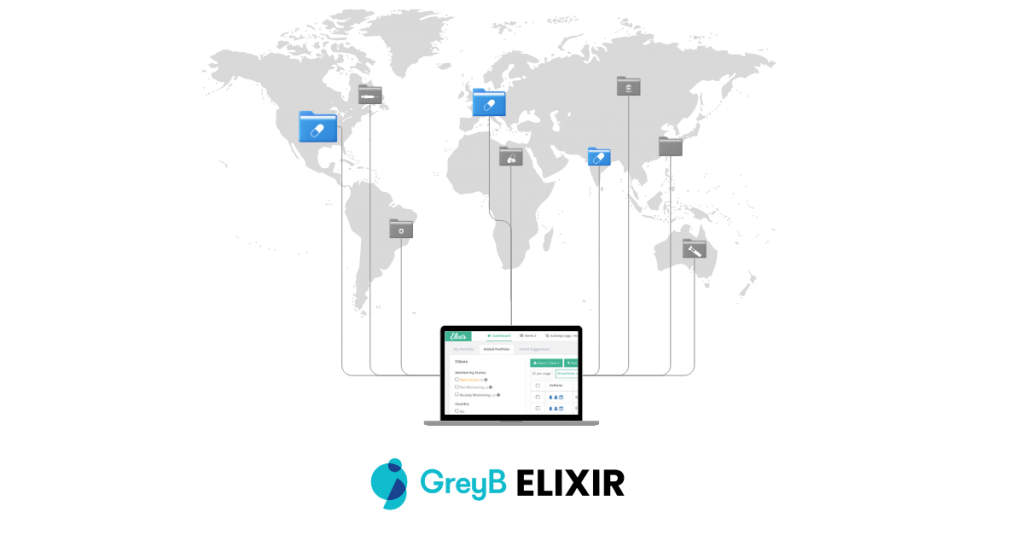
How many countries does Elixir covers
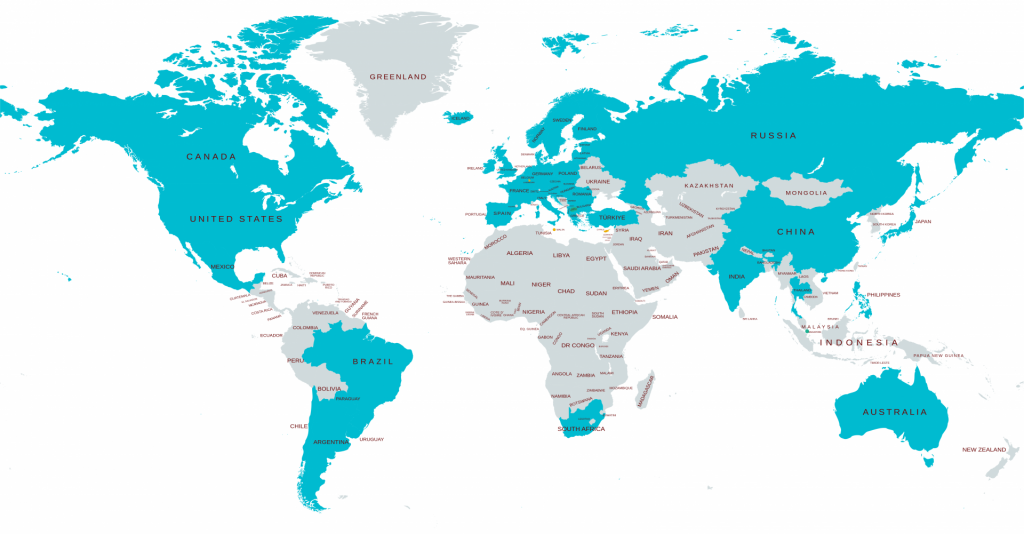
Elixir has helped companies streamline patent tracking
Elixir team provides a fast solution for any customisation”
|
Elixir team provides a fast solution for any customisation
|
Optimized notifications are useful to see all jurisdictions events
|
Most Asked Questions
How does a drug monitoring tool like Elixir work?
What types of information does Elixir track?
Who can benefit from using a drug monitoring tool like Elixir?
How does Elixir solve the problems associated with using spreadsheets?
Can Elixir be customized to fit specific business needs?
Yes, Elixir is a customizable platform that allows users to tailor their drug monitoring preferences and receive updates specific to their interests. It provides flexibility in tracking the information that is most relevant to their business or research activities.
How can Elixir help with making informed management decisions?
Is Elixir suitable for both bio-pharmaceutical and small-molecule drugs?
How does Elixir contribute to staying ahead of the competition?
How long do drug patents last?
Usually 20 years.
A drug patent expires 20 years from the date on which the patent application was filed in the United States. Sometimes, a term extension is provided if the original patent was delayed due to secrecy orders, interferences, or appellate review periods.
Generics can be launched once the patent-protected drug surpasses the patent expiry and exclusivity date.
Elixir provides the drug patents list expiring in a particular year, like 2023, 2024, and more.
Why is drug monitoring important?
What is your privacy policy on data? Is my data safe?
- We won’t use your data in any hidden ways for monetization or expansion activities. You own your data. We are ISO 27001 certified and SOC-2 compliant. Refer to our privacy policy.
- To ensure the safety of your data, we keep 3 months of backup in a geographical location to ensure no disaster can impact your data and operation.
Do I need to train my staff?
What are the geographical location of your servers?
What happens in case of any disaster?
How do I reach out to you in case of some query?
The support team is reachable at [email protected]; our response time is within 24 hours. You also get a dedicated support team with the package for faster response time.
