As a litigator, you always want to find that killer prior art that will eventually help your clients win an invalidity case, right?
But despite your best attempts, there may not always be a perfect Tier-I reference(s).
GreyB’s motto revolves around the fact that ‘IF it exists, we will find it’. The condition here is IF. But if 102 result does not exist, does it mean we give up?
NO. Instead, our strategy takes a different route which at times is to prove a ‘lack of inventive step’ based on obviousness. Hence, I present you with one such case where we proved the patent invalidity through obviousness using the research dissertation.
However, to understand this approach in depth, it is important that you see it in action. So let us begin.
Right from the beginning.
Shawn, a patent attorney, who handles various validity and infringement proceedings in a recognized US law firm, aimed to find 102 art for a patent that his client was infringing on. Therefore, he reached out to GreyB, and I was assigned his invalidation case related to the pharmaceutical formulation domain.
While studying the target patent, I noted the key details from it, such as it was on a slow-release pharmaceutical formulation of an active ingredient (let us call it drug A). Additionally, the formulation was a combination of two polymers, Polyethylene oxide (PEO) and poly(epsilon-caprolactone) (PCL). From the prosecution history of the target patent, I learned that the novel part of the subject invention is the specific molecular weight (MW) range of both the polymers used to manufacture the slow-release tablet. In a nutshell, the three requirements that a 102 art would have to fulfill were:
- Active pharmaceutical ingredient (drug A)
- Polyethylene oxide (PEO) with a molecular weight of 10,000 to about 200,000
- Poly(epsilon-caprolactone) (PCL) with a molecular weight of 10,000 to less than 1,000,000.
Please note – we can not disclose the drug information due to confidentiality.
Usually, when clients reach us, they have already tried looking for results on their own, and they might have faced some difficulty which eventually brings them to us. So I knew it wasn’t going to be full of challenges. And it was just as I thought!
[Challenge 1] Less Literature was published on the missing concept
From the preliminary analysis, I observed that Poly(epsilon-caprolactone) is one of the most commonly researched polymers in various implantable formulations. But when I started looking into the use of PCL in oral slow-release formulation, I came across some articles where the information mentioned in them investigated the oral administration of drugs other than the drug of our choice disclosed in the target patent.
Not just that, the literature I came across also lacked the molecular weight specifications and the formation’s first polymer, i.e., PEO.
Even after getting half-baked results, I searched the literature for another couple of days. When performing the citation analysis of the target patent using the Ambercite Tool, I found a reference (e.g., US20110251232A1) covering Polyethylene oxide aspects with the required MW range. With the past results that missed Polyethylene oxide, I was happy to finally find the mention of PEO.
However, even after getting a spot-on result with regard to PEOs, there was no mention of polycaprolactone (PCL)’s molecular weight.
At this point, it was normal to get frustrated as all the references I gathered had the required claim elements (i.e., Drug of our choice, PEO with required molecular weight, PCL) except one aspect, i.e., the molecular weight of PCL in such oral slow-release formulations.
Having invested so much effort and time into collecting these references, I managed to stay calm and started analyzing them in detail. I did not discard it because I knew that with such close references, they would yield some results. You may call this instinct or experience.
I observed that the molecular weight of PCL seemed to be an obvious feature. However, as per the patent’s prosecution history, the applicant had argued that the required molecular weight gave some physical characteristics to the tablet.
After exhausting the targeted search, I tweaked my strategy of looking for 102 art. On contemplating the results, I knew I required a prior art or a motivation to use the same polymers in the required molecular weight, providing the same properties irrespective of drugs.
Therefore, I broadened the search net to focus on PCL use irrespective of the active drug. I used different IPC/CPC classes specific to PCL to implement this search method. Additionally, I used different trade names for PCL, such as PURASORB or CAPROMER.
Despite my best efforts, I was unable to find any promising leads. At this point, I had to take a break from exploring dead ends in the patent literature.
What paved the way for the research dissertation?
I started exploring non-patent literature. Initially, while performing a keyword-based search on Google scholar, I found a research article by Dr. Lyons. I am mentioning this particular result because this was a ray of hope after days of dead ends.
As I evaluated the results, I kept Shawn and my mentor in the loop, sharing all of my findings with them.
Coming to the article by Dr. Lyons. It discussed the use of PCL to improve the stability of drug formulations. However, it lacked to disclose the role of PEO and the required drug in the same composition. Though I was again standing on a dead end, I took this author as the lead and started exploring the research article’s literature and citing references.
While exploring the same on the Google Scholar page for John G Lyons, 14 of his 72 articles were published before the cut-off date. Yet, his research articles were not exactly what I would call a bang-on result, as none mentioned a combination of PEO and PCL polymers in a single formulation. Most of them seemed to be fabricating hydrogels of materials other than PCL or PEO (not relevant).
During one of these explorations, I came across his dissertation. The dissertation outlined the use of PEO (required MW) and Tone 767 PCL blends in the production of monolithic matrices for oral drug delivery of carvedilol. This was the closest I had been to the required result. But the molecular weight of PCL was not explicitly disclosed in it.
You may think I would have been highly disappointed. Honestly, to some extent, I was. But at least this gave me the motivation to explore more dissertations from this author’s lab.
Hence, I started exploring the thesis on various websites like HOLLIS (Harvard Online Library Information System), DASH (Digital Access to Scholarship), EBSCO, OATD (Open Access Theses and Dissertations), DeepDyve, WorldCat, and Singapore Library, etc.)
And finally, my hard work smart work paid off as I got my hands on a Ph.D. dissertation where John G Lyons worked on melt-blended polymer-based monolithic matrices of PEO and PCL for controlled oral drug delivery.
In this case, the molecular weights of PEO and PCL fell in the required weight range. But that’s not it.
The author even laid an observation that the inclusion of PCL in the PEO/PCL blend makes them more processable, lowers the viscosity, and retards the drug profile.
Now I could scream BANG-ON! An exact match to the properties we were looking for.
But, before I could give out a sigh of relief, there was another challenge in front of me, i.e., a long document of approx. 600 pages which was not in a searchable format.
So to convert this uneditable form into an editable one, I first performed the Optical Character recognition of the document. Next, I studied the important parts like the abstract, results, summary, etc. I got the required excerpt after some reading when working on the document.
The next catch was that the active drugs mentioned were Carvedilol or Diclofenac instead of the required one. But at least I got the lead that a person skilled in the art can use it to combine two polymers with required weights with different drugs.
I decided to share the findings with Shawn and gather his perspective on this strategy of combining two types of results with the thesis to prove obviousness. It was a relief to know that he understood the motivation behind this strategy.
This was the first piece of positive return I got for the efforts, and all the time invested was worth it. After all, I got to think out of the box.
Although the combination and motivation approach was a go from Shawn’s side, the publication date for this thesis was another challenge.
[Challenge 2] Date confirmation for the research Dissertation
I have listed this under challenge because most of the websites (such as Science Direct, Research gate, and Google scholar) suggested its publication date to be after the cut-off date for our case (i.e., 2012).
If you wonder why I was still looking at other areas to confirm the publication date, then let me tell you that the PDF copy of the thesis displayed February 2007 (before our cut-off date) in its footprint. Therefore, there was inconsistency in the dates, and if the latter one is true, then I have a win-worthy result. But the PDF evidence was not that strong because neither web archives nor the site where this thesis was found confirmed the exact publication date.
The final strategy to extract the exact publication date
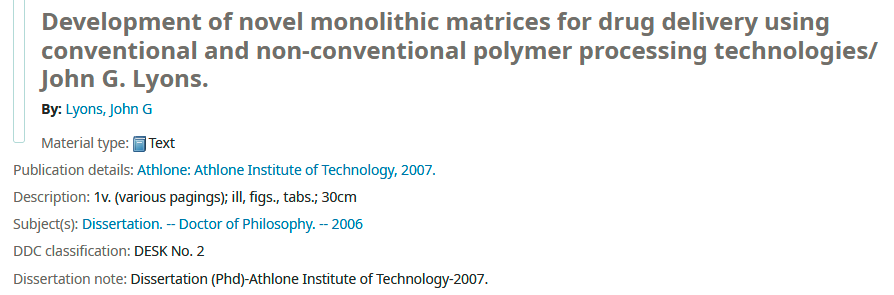
I was so close to getting the expected result, but the publication date was impeding it. Therefore to unwind the inconsistency in the dates, I checked the electronic libraries of Athlone Institute of Technology as the thesis was cataloged there.
I landed upon the web OPAC portal for the Technological University of the Shannon library, Athlone, Ireland, which has curated and cataloged their theses and confirmed that the one I found was publically available in 2007. Here is a snippet from the portal –
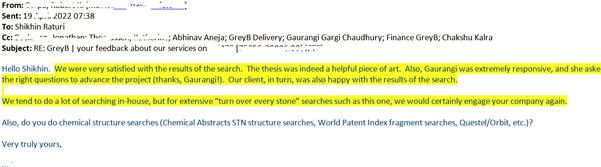
A win for the client and us. And definitely a big sigh of relief for me.
After all, what could be more satisfying than receiving such comments from the client after you deliver your final results?
Conclusion
No matter how much one wants to find 102 art, no amount of search strategies can land us there.
In such situations, the smart or rather winning way is to shift the search direction to explore the theses and books to find a 103 art. Through my years of experience, I have seen that this route can provide a strong motivation to combine results based on a reference’s weaker points.
Moreover, many litigators and patent attorneys prefer 102 or even 103 art found in books, research articles, and theses because the chances of their acceptance in court are very high.
Why not check out our other case studies if you found this case worthwhile?
How Patents Unmasked Alibaba’s Secret Partner?
How patent analytics enhanced the competitive analysis?
Authored by – Divyani (Team Lead, Life Sciences Department)