Approximately 85% of clinical trials fail to recruit enough participants, and 80% are delayed due to recruitment problems. High dropout rates further compromise the diversity and reliability of the trials. The clinical trials industry also has other persistent challenges, such as high costs, lengthy timelines, and fragmented data management.
Traditional methods often involve manual processes and lack comprehensive, real-time data integration. This leads to inefficiencies and high failure rates. Moreover, the under-representation of diverse populations in trials results in less generalizable outcomes.
Advanced technologies such as artificial intelligence, machine learning, digital health platforms, and decentralized trial designs address these issues.
These advancements reduce costs and timelines, enhance patient engagement, improve data accuracy, and ensure diverse participant inclusion. Moreover, these technological integrations streamline clinical trials, making them more efficient and effective.
This article highlights five innovative clinical trial startups offering unique solutions to modernize and improve the clinical trial processes.
They have the potential to grow rapidly, are in a good market position, and will introduce game-changing technology to the market in the next 2-3 years. This makes them a great option to partner, collaborate, or acquire.
Interested in knowing how the healthcare industry is changing with digital technologies like data analytics, AI, and connectivity technologies, and what new trends are shaping it. Our exclusive report on digital healthcare covers this and more details about innovations, startups, and companies.
Fill out the form below to get your copy of this report delivered to your inbox:

1. PhaseV
| Founding Year | 2023 |
| Headquarters | United States |
| Total Funding Amount | $15 Million |
| Last Funding Round/Amount | Seed/$15 Million |
| Website | https://phasevtrials.com/ |
Traditional clinical trial designs have significant challenges, such as high failure rates and inefficiencies. Clinical trials also face problems like the inability to adapt to real-time data, prolonged trial periods, increased costs, and lower success rates. These challenges significantly impact advanced-stage trials, like Phase 3 oncology trials, where stakes are high.
To combat these issues, PhaseV, a clinical trial startup, has developed an advanced platform that leverages adaptive trial designs and machine learning (ML) technologies. Its adaptive trial management system utilizes causal inference and reinforcement learning to dynamically adjust trial parameters based on ongoing data.
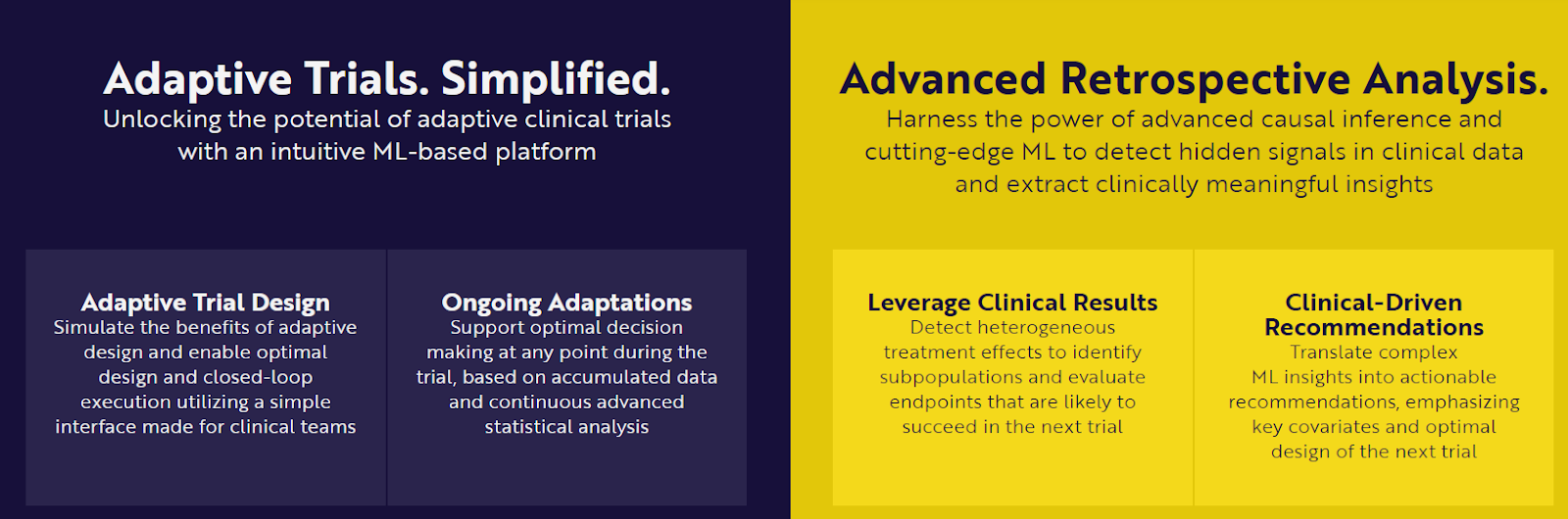
This system improves decision-making during trials, enhancing the ability to identify effective treatments more quickly. Additionally, their ML-powered analytics tools, such as the Subgroup Analysis Enhancer (SAE), are designed to detect heterogeneous treatment effects with greater precision than traditional methods.
PhaseV’s technology adaptive designs have been shown to reduce trial durations by up to 30% and increase the likelihood of success in Phase 3 trials by 20% compared to conventional trial designs. This innovative approach accelerates the development of new treatments and reduces costs and resource utilization.
CEO Raviv Pryluk, CTO Elad Berkman, and Dan Goldstaub co-founded this clinical trial startup.
Raviv served as a technological leader and commander in the Israel Defense Forces for more than 10 years. Before founding PhaseV, he was the Senior Vice President of Operations for Immunai. Raviv holds Ph.D. in computational neuroscience from the Weizmann Institute (Magna Cum Laude, John F. Kennedy Prize).
PhaseV’s latest seed funding round, for $15 million, was on 24 October 2023. The round, led by Exor Ventures and Viola Ventures, had five participants.
2. Yendou
| Founding Year | 2022 |
| Headquarters | Berlin, Germany |
| Total Funding Amount | €1.2 Million |
| Last Funding Round/Amount | Pre-seed/€1.2M Million |
| Website | https://www.yendou.io/ |
Manual processes and fragmented software systems are the primary causes of inefficiencies in clinical trial operations. These lead to significant delays and increased costs in clinical trials. Yendou, a Berlin-based startup, is tackling these inefficiencies in the pharmaceutical industry.
The startup has developed a clinical operations automation platform, often called a “Salesforce for R&D teams.” This platform streamlines data management, automates repetitive tasks, and consolidates information to expedite trial processes.
Yendou’s technology, the Clinical Operations Automation Platform (COAP), features a comprehensive CRM system designed specifically for life sciences R&D. It provides a curated pool of qualified clinical leads, CDA management, and automation of repetitive administrative tasks. The platform streamlines workflows and improves the data accuracy required for clinical trials.

The CDA management feature alone saves at least a week in trial tasks.
Compared to existing solutions, Yendou’s COAP offers a more integrated and automated approach, eliminating the need for manual data updates and disparate software systems.
CEO Zina Sarif and CTO Patrick Rogg co-founded this clinical trial startup.
Zina is a former biochemist with 10+ years of experience in cancer drug discovery. She also worked in clinical research finance management at Parexel for 3 years. Zina was also part of AstraZeneca for Phase III breast cancer studies. She completed her PhD in Biochemistry from the University of Witten/Herdecke.
Patrick was a software engineer for industry giants such as Citadel, Google, and AWS. He holds an MS in Computer Science from Hochschule München University of Applied Sciences.
Yendou raised its latest pre-seed funding round on Feb 28, 2024, for €1.2M Million. B2venture was the lead investor, and Meri Beckwith (founder of Lindus Health) was the angel investor for this round.
3. Mural Health
| Founding Year | 2022 |
| Headquarters | United States |
| Total Funding Amount | $20.73 Million |
| Last Funding Round/Amount | Seed/$12.7 Million |
| Website | https://www.anon.com/ |
Traditional clinical trials often suffer from high dropout rates, time-consuming recruitment processes, and low data integrity. These issues can delay new drugs’ entry into the market, costing sponsors between $600,000 and $8 million for each day a drug is delayed.
To solve these problems, Mural Health developed Mural Link, the first participant management platform. It is designed to simplify the clinical trial experience for participants and reduce the administrative burden for sites.
Mural Link provides various features, including flexible payment options without hidden fees, integrated travel arrangements via in-app ride-share services, and secure two-way messaging for better communication between participants and trial sites.
The clinical trial platform also offers real-time feedback mechanisms to improve participant retention and engagement.
Mural Link’s innovative approach has demonstrated significant improvements over traditional systems. The platform boosts compliance and retention rates by eliminating cumbersome processes and providing real-time data and analytics, leading to more efficient and successful clinical trials. This patient-centric approach has garnered positive feedback from users and industry experts alike.
Samuel Whitaker is the co-founder and CEO of Mural Health and an Angel Investor at Project Mayhem Ventures. He founded a similar clinical trial startup, Greenphire, and was its CEO and Board Member for 8 years. His work experience also includes working as CTO at Signant Health.
Mural Health raised its latest seed funding of $12.7 Million on 5 April 2024.
4. Macro Trials
| Founding Year | 2021 |
| Headquarters | United States |
| Total Funding Amount | $6 Million |
| Last Funding Round/Amount | Seed/$4.5 Million |
| Website | https://www.macrotrials.com/ |
The high failure rates of clinical trials are often due to inadequate enrollment and the under-representation of diverse populations. These issues lead to increased costs and extended timelines for drug development, with an estimated $2.7 billion required to bring a high-value therapeutic to market, and 70% of that cost attributed to clinical trials.
To address these challenges, Macro Trials has developed a precision research clinical platform that leverages a distributed “hub-and-spoke” model to conduct trials in various settings, from in-person to decentralized formats.
This platform includes workflow-native efficiency tools, a network of logistic partners, and digital infrastructure for real-time data collection and management. Macro’s platform supports all trial designs, enabling physician participation in groundbreaking research and delivering industry-best enrollment and retention rates. The startup’s approach lowers the barriers to entry for clinical research, driving equitable enrollment that reflects real-world patient demographics.
Macro Trials differentiates itself from traditional clinical trial methods by focusing on including diverse populations and generating real-world evidence (RWE). Its trials have shown significantly higher diversity, with over 75% female and 46% Hispanic participants in recent studies, compared to 41% and 6% industry averages.
Iana Dimkova (CEO), Babak Azizzadeh, and Jonathan Cabin (CMO) co-founded Macro Trials.
Iana is also a co-founder of Initiate Ventures & Studio, which co-creates and invests in healthcare and medical technology startups. She has experience as a board member and advisor for Vineti, Evidation Health, Genome Medical, Inc., and Gravie.
Jonathan Cabin holds a Doctor of Medicine (MD)from the Yale School of Medicine. He is a Facial Plastic & Reconstructive Surgeon at SkinDC and a Staff Physician in multiple Hospitals. Jonathan also co-founded Docola, a social organization for free asynchronous care communication platform.
Macro Trials raised its latest seed funding round for $6M on Jun 21, 2023. MBX Capital was the lead investor for this round. Participants include INITIATE Ventures (the incubator for Macro Trials), Healthy VC, Inflect Health, and Village Global, a venture firm backed by Jeff Bezos, Bill Gates, and Mark Zuckerberg.
Related Read
5. CitrusLabs
| Founding Year | 2016 |
| Headquarters | United States |
| Total Funding Amount | $5.34 Million |
| Last Funding Round/Amount | Seed/Publicly Not Disclosed |
| Website | https://www.citruslabs.com/ |
Traditional clinical trials often involve high costs and complex processes, making it difficult for smaller brands to validate their product claims. Citruslabs aims to simplify this process by offering budget-friendly, comprehensive clinical trial services that do not compromise quality or scientific rigor.
The startup has developed a full-service Contract Research Organization (CRO) platform integrating advanced technology with deep industry expertise. The platform supports various virtual, site-based, and hybrid trials, allowing flexibility and efficiency.
Citruslabs’ services include study design, patient recruitment, data collection, and analysis, all designed to accelerate trial timelines and reduce costs. Its approach ensures precision in every study, helping brands substantiate their product claims with robust scientific data.
The startup helps conduct trials in multiple industries, including skincare, biotechnology, medical devices, and supplements. A few of its clients include brands like NakedPoppy, Lazarus Naturals, Semaine, Your Super, and Oxford VR.
Citruslabs utilizes a vast database for efficient patient recruitment and retention, ensuring high compliance and successful trials. Furthermore, it provides clients full access to the data collected during trials, allowing brands to use this information for marketing, regulatory submissions, and more. This transparent and customer-centric approach has enabled Citruslabs to help brands like Semaine achieve significant business growth through validated product claims and increased market credibility.
CEO Susanne Mitschke, Patrick Renner (COO), and Rogelio Arellano (CTO) co-founded this clinical trial startup. These three are also founders of MindMate (Techstars NYC ’16), an app to reduce the risk of cognitive decline.
Susanne started her career with PwC as a Junior Consultant. She holds a Master of Public Health – MPH in Epidemiology and Biostatistics from Harvard T.H. Chan School of Public Health.
CitrusLabs raised a total funding of $5.3 Million over 7 funding rounds. The latest seed funding round was on 16 May 2019.
Partner with growth-stage innovative clinical trial startups working on your industry’s toughest challenges and stay ahead of the competition.
Learn how GreyB helps you discover similar ventures that perfectly fit your needs.
How Can We Help You?
We support industry-leading R&D and Innovation professionals through complex problems. Describe your challenge, and let us bring clarity and expertise.